Alpha Globins Family
This is a case study that was analyzed in one of our articles where we generalize the use of FrustraEvo to study protein families stability and functional patterns: Freiberger et al Nature Comms 2023.
In the following link you can find a FrustraEvo reproduction of this example:
FrustraEvo job results
Biological Interpretation:
In total, there are 12 highly frustrated positions in α globins. This points out at functional adaptations that have happened over the evolutionary history of alpha globins. Several of these loci correspond to residues involved with the asymmetric interactions of alpha globins in the context of the tetrameric structure of hemoglobin i.e. K39α, and Y42α.
Other highly frustrated residues correspond to the differential function and structural details of each subunit type, e.g., K99α and S124α interact with the αHb-stabilizing protein (AHSP), a chaperone that prevents α-globin toxicity when isolated. In addition, K7α, E27α, and E30α form intra-subunit salt bridges that are critical for allostery and the Bohr effect.
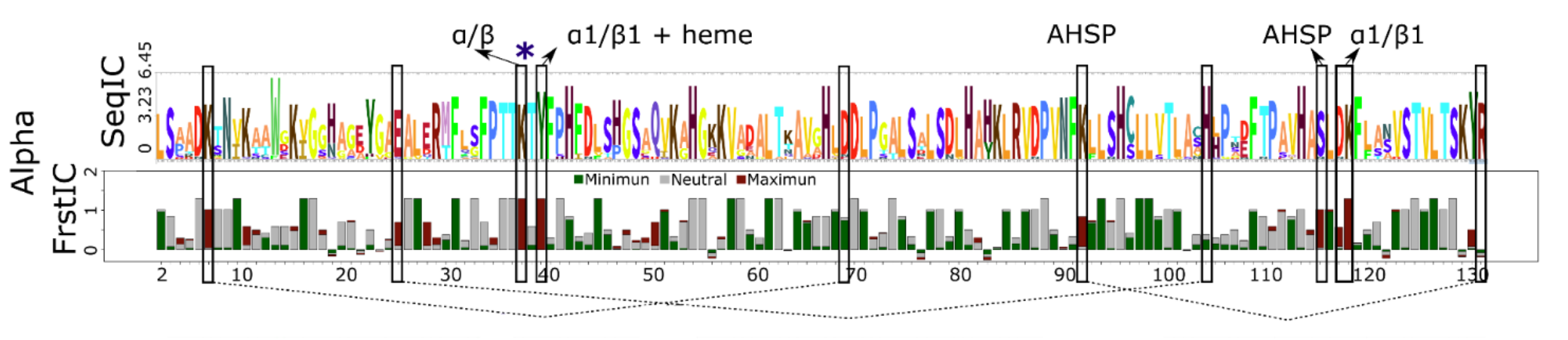
Arrows above the logo point at residues involved in protein-protein interactions. Dashed lines connect residues involved in salt bridges.